For my girls in 6-1, 6-2 and 6-3:
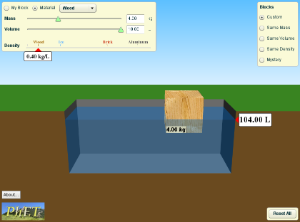
1)Click on the simulation below. Then on the right side, click "Mystery" button. Use the scale and the water to measure the densities of each block A-E, and compare them to known values in the table. Write down what you think each block is made of on your paper, and check your answers with a neighbor before going to the next simulation.
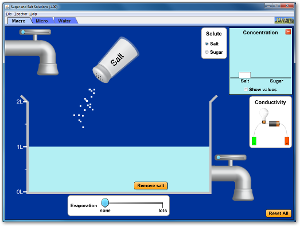
2) Click on the simulation below. Then click on the tab, "Micro" and shake some sodium chloride into the water. Answer the following questions in your composition book.
A. What does the sodium chloride look like before it enters the water?
B. What happens to the sodium chloride crystals after they enter the water?
C. What do the purple dots represent? What do the green dots represent?
D. What can you do to increase the concentration of the salt water? (Make it saltier?)
E. What can you do to decrease the concentration of the salt water? (Make it less salty?)
F. Click "remove solute" and change the solute to sucrose (a type of sugar similar to glucose). Describe what the sucrose looks like before it hits the water.
G. What happens to the sucrose molecules after it enters the water? How is this different from the sodium chloride?
H. Click on the tab "Water," and see what happens on a smaller scale when you add sodium chloride and sucrose to water. What colors represent the water molecules?
I. Why do the all the molecules seem to move around so much?
J. What do you think would happen to the speed of the molecules if we heated the water? Why do you think this?
K. Click back to the "Macro" tab and experiment with the conductivity device (the lightbult and battery). Talk to a classmate and come up with an explanation of why you think the salt solution will light the lightbulb but the sugar solution will not.
Check all your answers with a classmate before you finish. Turn in your paper when you are done.
Have fun!
No comments:
Post a Comment